Catalytic ozonation for degradation of persistent organic pollutants (POPs) in wastewater
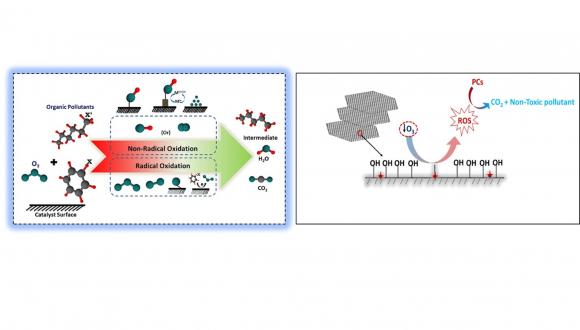
Ozonation, due to its ease of implementation at full scale, broadband action against various classes of POPs, is one most adapted AOPs as a tertiary treatment applied in wastewater reclamation. Oxidation of PrCs by ozonation is achieved by direct oxidation of molecules by ozone or indirect oxidation via Reactive Oxygen Species (ROS) produced by the ozone decomposition. Ozonation technology has been used as a disinfectant for several decades, and in recent years it is also being used to abate persistent organic pollutants (POPs) in secondary wastewater effluents. Many countries such as the USA, Switzerland, France, Austria, Germany employ ozone as secondary or tertiary wastewater treatment. These facilities use ozone alone or in combination with UV or membrane processes for disinfection and treating micropollutants in wastewater. Nevertheless, the economically relevant ozone doses do not mineralize the compound. Instead, various transformation products are formed—some transformed products found to be more toxic and less biologically active than the parent compound.
Our laboratory focused on identifying PrCs in wastewater and adapting various technologies from the past decade. We found cyclophosphamide (CYP), iohexol (IHX), valsartan (VAL), lamotrigine (LMG), sulfamethoxazole (SMX), bezafibrate (BZF) in wastewater at concentrations ranging from ppb-ppm. Ozonation and peroxonation (O3/H2O2) have been adapted to degrade the same in distilled water (DI) and simulated wastewater, and the results suggested that even though ozonation and peroxonation did not degrade, the compounds instead transformed to be more toxic and less biodegradable than the parent compound in some cases. These studies imply that conventional AOPs are not ideal for treating secondary or tertiary treatment of wastewater. Besides inefficient mineralization of pollutants, less solubility and stability of ozone pose limitations for applicability of ozone in wastewater treatment plants. Heterogonous catalytic ozonation overcomes these limitations via enhancing the catalytic decomposition of ozone-producing ROS. Furthermore, the catalyst should be cheap for real-time applications, abundant in the environment, and not cause secondary pollution.
Objectives:
- Synthesis and characterization of catalyst based on nanomaterials
- Studying the effect of ozonation and catalytic ozonation on the degradation of POPs and TPs.
- Understanding the mechanism of degradation of POPs by proposed catalysts
- Fabrication of catalyst into scaffolds by 3D printing.